Ho phaella ho thekenoloji, motsoako oa glycosides esale o thahasella saense, kaha ke karabelo e tloaelehileng haholo tlhahong.Lipampiri tsa morao-rao tse ngotsoeng ke Schmidt le Toshima le Tatsuta, hammoho le litšupiso tse ngata tse boletsoeng ho eona, li buile ka mefuta e mengata e mengata ea lintho tse ka etsoang ka maiketsetso.
Ka har'a motsoako oa li-glycosides, likaroloana tse nang le tsoekere e ngata li kopantsoe le li-nucleophile, joalo ka li-alcohol, lik'habohaedreite kapa liprotheine, haeba karabelo e ikhethileng e amanang le e 'ngoe ea lihlopha tsa hydroxyl ea carbohydrate e hlokahalang, mesebetsi e meng kaofela e tlameha ho sireletsoa. mohato oa pele.Ha e le hantle, lits'ebetso tsa enzymatic kapa microbial, ka lebaka la khetho ea tsona, li ka nka sebaka se rarahaneng sa ts'ireletso ea lik'hemik'hale le mehato ea ts'ireletso ho khetha ho tloha ho glycosides libakeng.Leha ho le joalo, ka lebaka la nalane e telele ea li-alkyl glycosides, tšebeliso ea li-enzyme ka har'a motsoako oa glycosides ha e so ithutoe le ho sebelisoa haholo.
Ka lebaka la bokhoni ba litsamaiso tsa li-enzyme tse loketseng le litšenyehelo tse phahameng tsa tlhahiso, motsoako oa enzymatic oa alkyl polyglycosides ha o e-so lokele ho ntlafatsoa ho ea boemong ba indasteri, 'me mekhoa ea lik'hemik'hale e khethoa.
Ka 1870, MAcolley e ile ea tlaleha ho qaptjoa ha "acetochlorhydrose"(1,figure2) ka karabelo ea dextrose(glucose) e nang le acetyl chloride, e ileng ea qetella e lebisitse nalaneng ea litsela tsa glycoside synthesis.
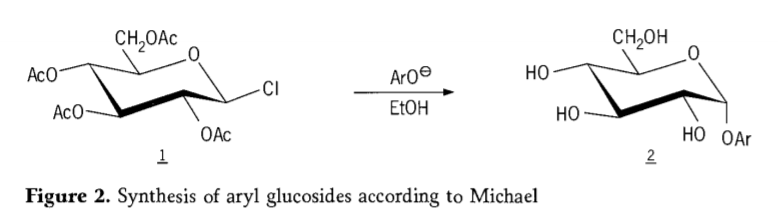
Tetra-0-acetyl-glucopyranosyl halides(acetohaloglucoses) hamorao ho ile ha fumanoa e le li-intermediate tse molemo bakeng sa motsoako oa stereoselective oa li-alkyl glucosides tse hloekileng.Ka 1879, Arthur Michael o ile a atleha ho lokisa li-aryl glycosides tse hlakileng, tse ka khonang ho tsoa ho li-intermediate tsa Colley le phenolate.(Aro-, Setšoantšo sa 2).
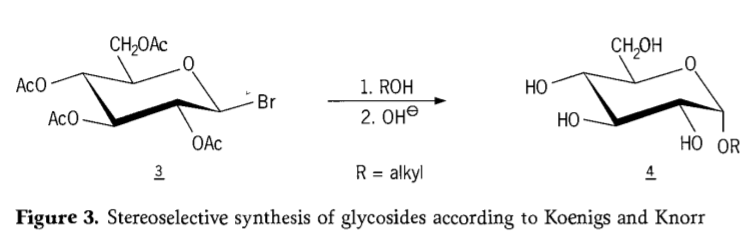
Ka 1901, tlhahiso ea Michael ho mefuta e mengata e fapaneng ea lik'habohaedreite le hydroxylic aglycon, ha W.Koenigs le E.Knorr ba ne ba hlahisa mokhoa oa bona o ntlafetseng oa stereoselective glycosidation (Setšoantšo sa 3).Boitšoaro bo kenyelletsa phetoho ea SN2 ka carbon ea anomeric 'me e tsoela pele ka mokhoa o tsitsitseng ka ho fetoha ha tlhophiso, ho hlahisa mohlala α-glucoside 4 ho tloha ho β-anomer ea aceobromoglucose intermediate 3. Koenigs-Knorr synthesis e etsahala ka pel'a silevera kapa silevera kapa bahlahisi ba mercury.
Ka 1893, Emil Fischer o ile a etsa tlhahiso ea mokhoa o fapaneng haholo oa ho kopanya li-alkyl glucosides.Ts'ebetso ena e se e tsejoa e le "Fischer glycosidation" mme e kenyelletsa karabelo ea acid-catalyzed ea glycoses le joala.Ak'haonte efe kapa efe ea nalane e tlameha ho kenyelletsa teko ea pele e tlalehiloeng ea A. Gautier ka 1874, ea ho fetola dextrose ka anhydrous ethanol boteng ba hydrochloric acid.Ka lebaka la tlhahlobo e fosahetseng ea mantlha, Gautier o ne a lumela hore o fumane "diglucose".Hamorao Fischer o ile a bontša hore "diglucose" ea Gautier ha e le hantle e ne e le ethyl glucoside (Setšoantšo sa 4).
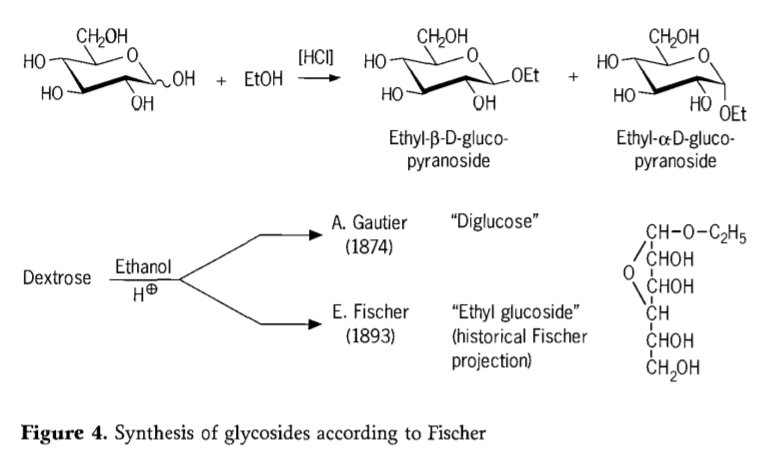
Fischer o hlalositse sebopeho sa ethyl glucoside ka nepo, joalo ka ha ho ka bonoa ho tsoa ho foromo ea nalane ea furanosidic e sisintsoeng.Ha e le hantle, lihlahisoa tsa Fischer glycosidation li rarahane, haholo-holo metsoako e leka-lekaneng ea α/β-anomers le pyranoside/furanoside isomers eo hape e nang le li-oligomers tsa glycoside tse kopantsoeng ka mokhoa o sa reroang.
Ka hona, mefuta e meng ea limolek'hule ha ho bonolo ho arola metsoako ea Fischer reaction, eo esale e le bothata bo boholo nakong e fetileng.Ka mor'a ntlafatso e itseng ea mokhoa ona oa ho kopanya, Fischer hamorao o ile a amohela motsoako oa Koenigs-Knorr bakeng sa lipatlisiso tsa hae.E sebelisa ts'ebetso ena, E.Fischer le B.Helferich e bile bona ba pele ba tlalehang ho kopanngoa ha alkyl glucoside ea ketane e telele e bonts'ang thepa ea surfactant ka 1911.
Ho tloha ka 1893, Fischer o ne a hlokometse ka nepo lintho tsa bohlokoa tsa alkyl glycosides, joalo ka botsitso ba tsona bo phahameng mabapi le oxidation le hydrolysis, haholo mecheng ea litaba tsa alkaline e matla.Litšobotsi tsena ka bobeli li bohlokoa bakeng sa alkyl polyglycosides lits'ebetsong tsa surfactant.
Lipatlisiso tse amanang le karabelo ea glycosidation li ntse li tsoela pele 'me litsela tse' maloa tse khahlisang tsa glycosides li entsoe nakong e fetileng.Tse ling tsa mekhoa ea ho kopanya li-glycosides li akaretsoa setšoantšong sa 5.
Ka kakaretso, lits'ebetso tsa glycosidation tsa lik'hemik'hale li ka aroloa ka lits'ebetso tse lebisang ho rarahaneng ea oligomer equilibria ka phapanyetsano ea acid-catalysed glycosyl.
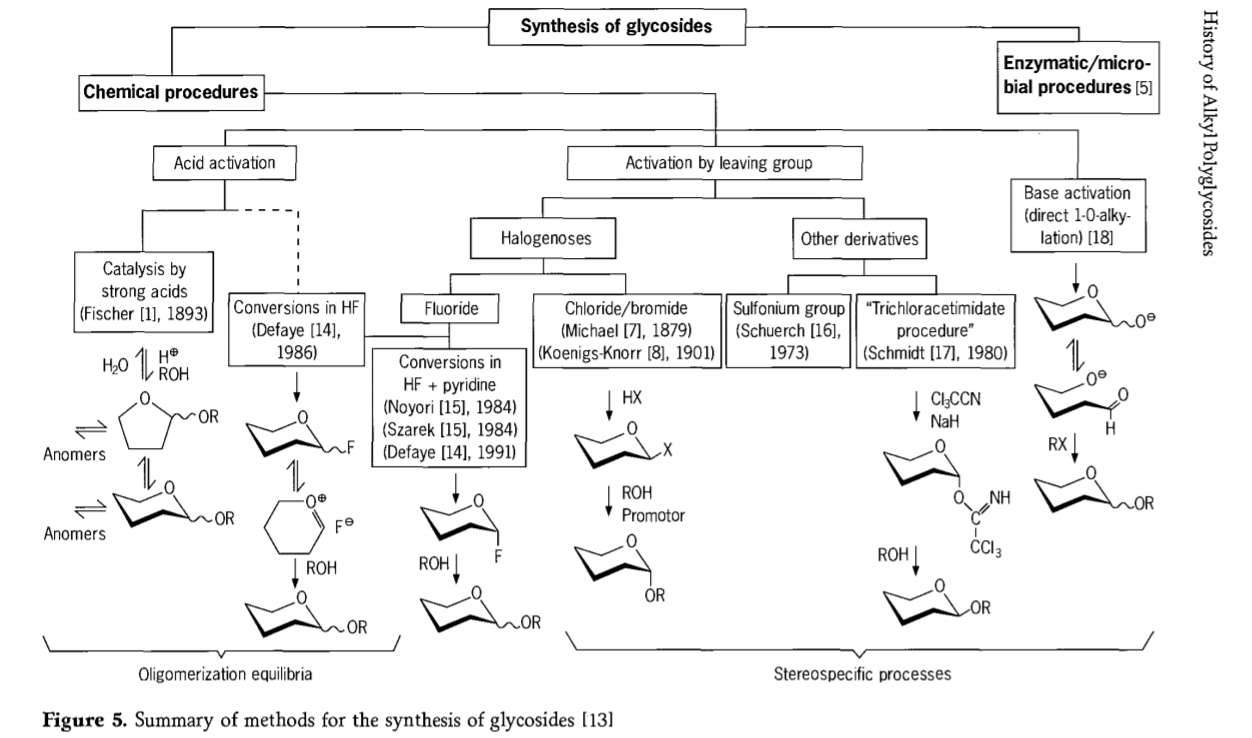
Karabelo ho li-substrates tsa lik'habohaedreite tse kentsoeng ka nepo (karabelo ea Fischer glycosidic le hydrogen fluoride(HF) e nang le limolek'hule tsa lik'habohaedreite tse sa sireletsoang) le karabelo ea kinetics e laoloang, e ke keng ea khutlisetsoa morao, 'me haholo-holo e nka sebaka sa stereotaxic.Mofuta oa bobeli oa ts'ebetso o ka lebisa ho thehoeng ha mofuta o mong ho e-na le metsoako e rarahaneng ea karabelo, haholo ha e kopantsoe le mekhoa ea lihlopha tsa paballo.Li-carbohydrate li ka siea lihlopha holim'a carbon ea ectopic, joalo ka liathomo tsa halogen, sulfonyls, kapa trichloroacetimidate lihlopha, kapa li kenngoe ke li-bases pele li fetoloa ho triflate esters.
Boemong bo itseng ba glycosidations ho hydrogen fluoride kapa metsoako ea hydrogen fluoride le pyridine (pyridinium poly [hydrogen fluoride]), glycosyl fluoride e thehoa ka situ mme e fetoloa hantle hore e be glycosides, mohlala ka joala.Hydrogen fluoride e ile ea bontšoa e le mokhoa o matla oa ho sebetsa, o sa nyenyefatse;equilibrium auto condensation(oligomerization) e bonoa e ts'oana le ts'ebetso ea Fischer, le hoja mokhoa oa ho arabela mohlomong o fapane.
Li-alkyl glycosides tse hloekileng ka lik'hemik'hale li loketse feela lits'ebetso tse ikhethang haholo.Mohlala, li-alkyl glycosides li sebelisitsoe ka katleho lipatlisisong tsa biochemical bakeng sa kristallise ea liprotheine tsa membrane, joalo ka kristallise ea likarolo tse tharo tsa porin le bacteriorhodopsin boteng ba octyl β-D-glucopyranoside (liteko tse ling tse thehiloeng mosebetsing ona li lebisa ho Nobel). khau ea k'hemistri bakeng sa Deisenhofer, Huber le Michel ka 1988).
Nakong ea nts'etsopele ea alkyl polyglycosides, mekhoa ea stereoselective e 'nile ea sebelisoa lekaleng la laboratori ho kopanya mefuta e fapaneng ea lintho tsa mohlala le ho ithuta thepa ea bona ea physicochemical, ka lebaka la ho rarahana ha bona, ho se tsitse ha li-intermediates le palo le tlhaho ea bohlokoa ea ts'ebetso. li-wasters, li-syntheses tsa mofuta oa Koenigs-Knorr le mekhoa e meng ea lihlopha tse sireletsang li ka baka mathata a bohlokoa a tekheniki le moruo.Mekhoa ea mofuta oa Fischer ha e thata haholo ebile e bonolo ho e etsa ka tekanyo ea khoebo, ka hona, ke mokhoa o ratoang oa ho hlahisa li-alkyl polyglycosides ka bongata.
Nako ea poso: Sep-12-2020





